Services
Customized Proteins
At CNETE, we offer a comprehensive service for the design and production of specialized proteins tailored to your needs. Our expertise covers the entire development cycle, from proof of concept to industrial validation, with cutting-edge skills in: At CNETE, we offer a comprehensive service for the design and production of specialized proteins tailored to your needs. Our expertise covers the entire development cycle, from proof of concept to industrial validation, with cutting-edge skills in
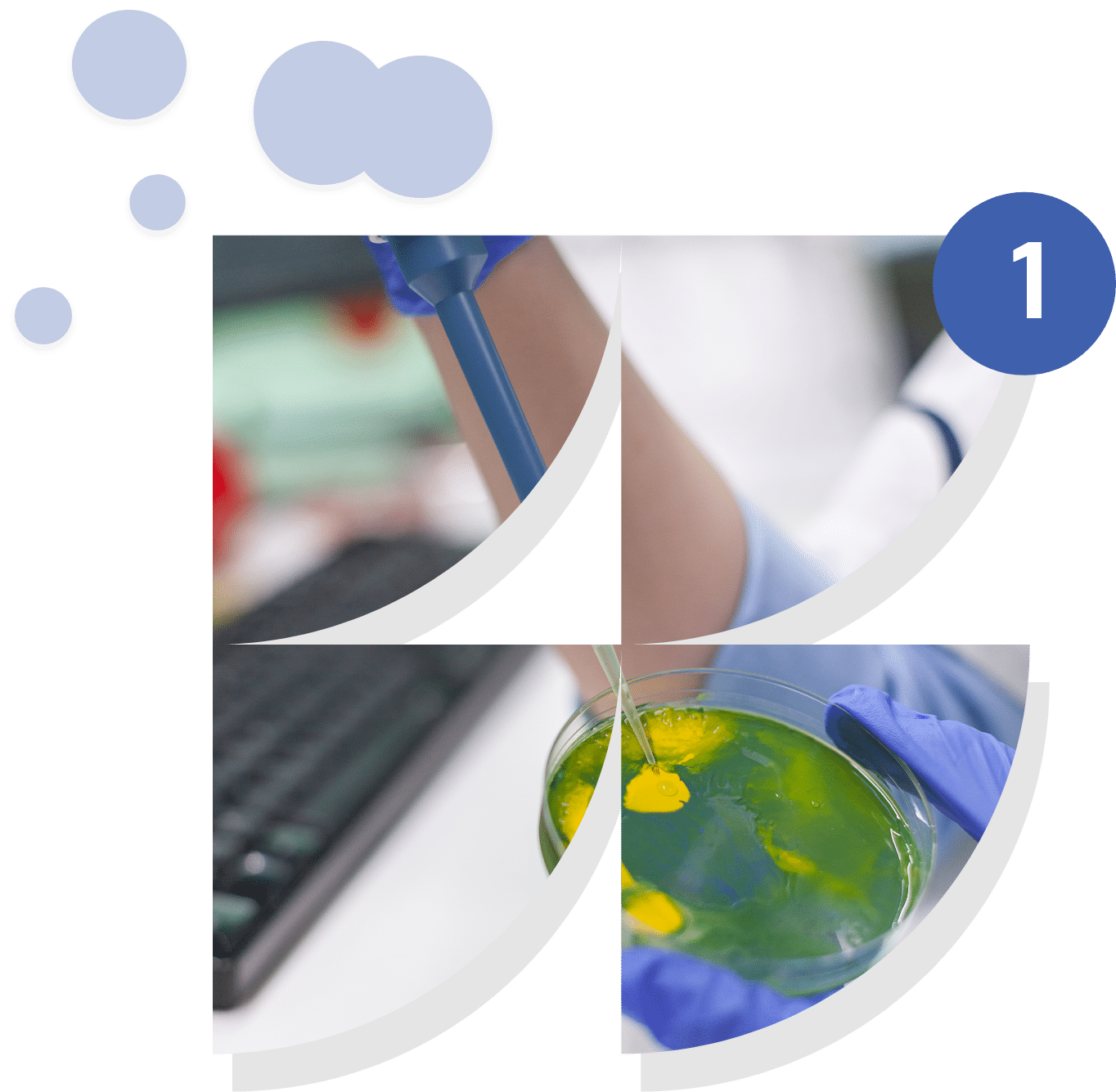
Microbial Engineering
and Proof of Concept
This component focuses on the creation of genetically modified microorganisms to optimize specific functions, such as the production of biomolecules of interest or the degradation of targeted compounds. The approach includes rational design, genome editing, and experimental validation in the laboratory through proof-of-concept trials. The objective is to demonstrate scientific and technical feasibility before moving to industrial or applied scale, while complying with biosafety and ethical standards.
Bioprocess optimization
This step aims to define and adjust key culture parameters—such as temperature, pH, nutrient concentration, aeration, and agitation—in order to maximize the efficiency and yield of microorganisms or biological systems. Through controlled trials and modeling approaches, the goal is to identify the optimal conditions for targeted production, while ensuring process stability, product quality, and reproducibility at scale.

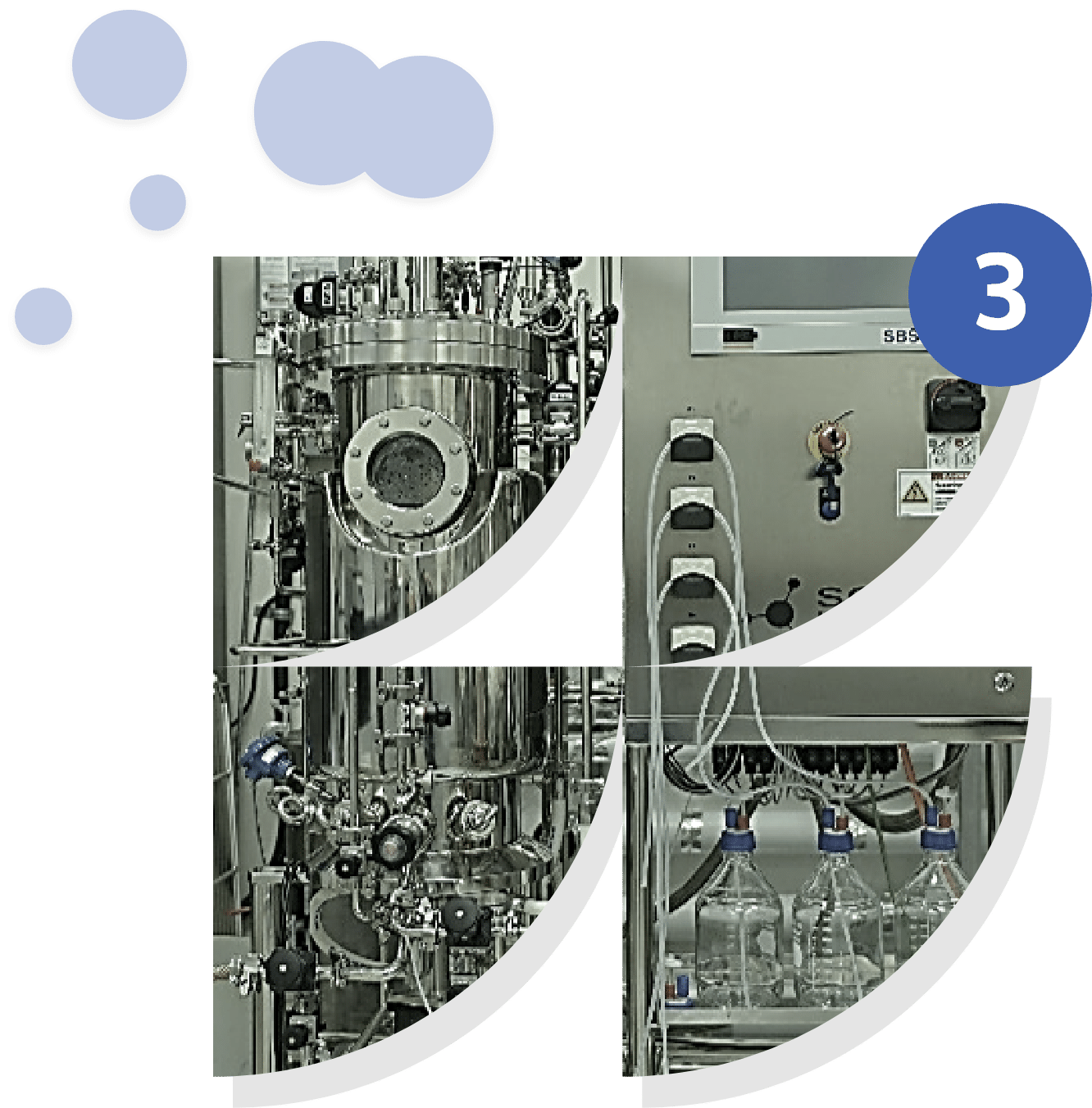
Scaling up in a bioreactor
This phase involves transferring validated processes from the laboratory to larger volumes, ranging from pilot production (1 L) to intermediate-capacity bioreactors (700 L). The aim is to test the robustness and reproducibility of the process under conditions closer to industrial scale. This includes adjusting culture parameters, managing oxygenation and temperature control, and monitoring the growth and productivity of microorganisms. This step makes it possible to anticipate the technical constraints associated with industrialization and ensure consistent quality in the final product
Purification and formulation
At this stage, the proteins obtained after fermentation are isolated and purified using appropriate techniques (filtration, chromatography, precipitation, etc.) in order to remove impurities and achieve a high degree of purity. Formulation then stabilizes these proteins and prepares them in a standardized form, whether as a solution, freeze-dried powder, or other presentation depending on the intended application. This phase is essential to ensure the reproducibility, safety, quality, and efficacy of biomolecules, while laying the groundwork for preclinical, clinical, or industrial testing

Industrial transfer and validation
This final stage aims to adapt the production process to industrial constraints in order to ensure large-scale, stable, and economically viable manufacturing. It includes process validation, equipment qualification, and the implementation of protocols that comply with good manufacturing practices (GMP). Compliance with national and international regulatory standards is therefore essential to guarantee the safety, efficacy, and traceability of the product. This transfer to industry marks the transition from proof of concept in the laboratory to market launch, paving the way for regulated and sustainable commercial exploitation
Cost assessment and profitability
This phase involves analyzing in detail all costs associated with the production process—raw materials, energy, labor, equipment, maintenance, and regulatory compliance—in order to establish a realistic economic model. A profitability study is conducted to compare the required investments and potential revenues, taking into account scaling scenarios and market fluctuations. This economic validation makes it possible to determine the commercial viability of the project, guide strategic choices, and identify optimization levers before making a final commitment to industrial production.

State-of-the-Art Facilities
From proof of concept to production: $13M in equipment for your ambitions!
150 state-of-the-art instruments to drive your innovations, from lab scale to pilot scale.
Two strategic sites totaling 1,370 m², including 847 m² dedicated to laboratories, to turn your ideas into concrete solutions.

Bioreactors from 1L to 700L
The use of bioreactors with progressive capacities ranging from 1 L to 700 L is a key step in ensuring the transition from processes developed in the laboratory to their application on an industrial scale. This increase in volume makes it possible to evaluate the stability of the process, adapt critical parameters (oxygenation, agitation, pH, temperature), and test the robustness of microorganisms or biological systems under conditions close to those found in industrial settings. These intermediate tests ensure better control of performance before full industrialization, while reducing the technical and economic risks associated with scaling up.
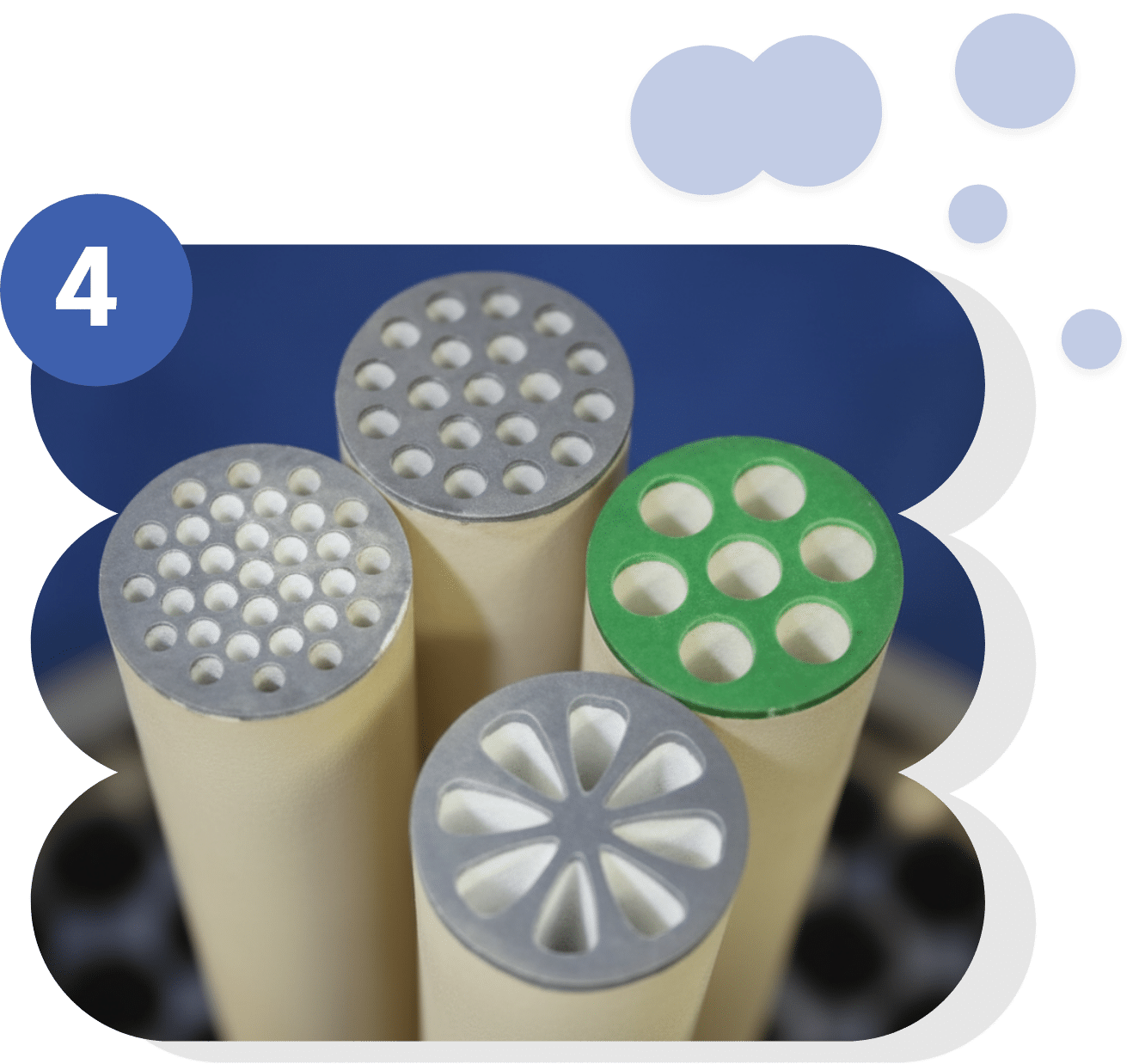
Advanced purification technologies
The use of advanced purification technologies is essential for obtaining high-quality biomolecules that meet the required purity and safety standards. Tangential filtration allows for the effective separation of proteins and the concentration of solutions without denaturing them. Liquid chromatography, meanwhile, offers high precision in the removal of impurities and the selection of active fractions. Finally, freeze-drying ensures long-term stabilization of products by transforming them into powder while preserving their functional integrity. The integration of these approaches guarantees a standardized, reproducible end product that is ready for clinical or industrial applications.


Cutting-edge analytical capabilities
Advanced analytical tools ensure accurate product evaluation. Enzymatic monitoring controls biological activity, stability analysis measures shelf life and robustness, while protein characterization (mass spectrometry, electrophoresis, NMR) provides essential information on their structure and properties. These approaches guarantee the quality, safety, and compliance of biomolecules before their clinical or industrial use.
Protein Characterization
Platform
The characterization platform enables in-depth analysis of the proteins produced in order to assess their purity, structure, and functionality. Using advanced analytical tools, it provides detailed monitoring of biomolecule performance throughout the production process. These analyses ensure consistent quality, compliance with regulatory standards, and the reliability of products intended for clinical or industrial applications.

